Removal of Cu(II) and Pb(II) Ions from Wastewater Solutions Using Black Soldier Fly (Hermetia illucens) Pupal Shell: Adsorption and Characterization
Abstract
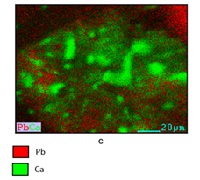
Industrial wastewater often contains heavy metals such as Pb(II) and Cu(II) that pose significant environmental and health risks. This study investigates the utilization of Black Soldier Fly (BSF) (Hermetia illucens) pupal shells as an adsorbent material for the removal of Pb(II) and Cu(II) ions from aqueous solutions. BSF pupal shells were chosen due to their high availability, rapid life cycle, and chitin-rich composition, making them suitable for heavy metal adsorption. The preparation process included washing, drying, grinding, and activation with 1 M NaOH solution. Characterization of the adsorbent was performed before and after adsorption using Fourier Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy coupled with Energy Dispersive X-ray Spectroscopy (SEM-EDX). Adsorption experiments were conducted to examine the effects of pH, contact time, and initial ion concentration. The optimum pH for adsorption was found to be 5.5, achieving removal efficiencies of 95.5% for Pb(II) and 71.81% for Cu(II). The optimum contact times were 180 minutes for Pb(II) and 240 minutes for Cu(II). Kinetic analysis demonstrated that the adsorption process followed a pseudo-second-order model. Adsorption isotherm studies indicated that the Langmuir model provided a better fit (R² = 0.99 for Pb(II) and 0.98 for Cu(II)) compared to the Freundlich model (R² = 0.90 for Pb(II) and 0.77 for Cu(II)). These results demonstrate that BSF pupal shells are a promising, cost-effective, and environmentally friendly material for industrial wastewater treatment applications
Keywords
Full Text:
PDFReferences
[1] A. Yusfaddillah, R. D. Saputri, T. W. Edelwis, dan H. Pardi, “Heavy metal pollution in Indonesian waters,” BIO Web of Conferences, vol. 79, p. 04001, 2023. doi: 10.1051/bioconf/20237904001
[2] N. Fahimah, I. R. S. Salami, K. Oginawati, S. H. Susetyo, A. Tambun, A. N. Ardiwinata, dan S. Sukarjo, “The assessment of water quality and human health risk from pollution of chosen heavy metals in the Upstream Citarum River, Indonesia,” Journal of Water and Land Development, vol. 56, pp. 153–163, 2023. doi: 10.24425/jwld.2023.143756
[3] S. Rajoria, M. Vashishtha, dan V. K. Sangal, “Treatment of electroplating industry wastewater: a review on the various techniques,” Environmental Science and Pollution Research, vol. 29, no. 48, pp. 72196–72246, 2022. doi: 10.1007/s11356-022-18643-y
[4] T. Aragaw, “Chromium Removal from Electroplating Wastewater Using Activated Coffee Husk Carbon,” Adsorption Science & Technology, vol. 2022, Article ID 7646593, 2022. doi: 10.1155/2022/7646593
[5] L. Weerasundara, Y. S. Ok, dan J. Bundschuh, “Selective Removal Of Arsenic In Water: A Critical Review,” Environmental Pollution, vol. 268, p. 115668, 2021. doi: 10.1016/j.envpol.2020.115668
[6] M. Uddin dan Y. Jeong, "Efficiently performing periodic elements with modern adsorption technologies for arsenic removal," Environmental Science and Pollution Research, vol. 27, no. 32, pp. 39888–39912, 2020. doi: 10.1007/s11356-020-10323-z.
[7] S. Manoj, S. S. Patil, dan M. R. Naik, "Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review," Journal of Environmental Management, vol. 254, p. 109779, 2020. doi: 10.1016/j.jenvman.2019.109779.
[8] J. Thilagan, S. Goplakrishnan, dan T. Kannadasan, "Continuous Fixed Bed Column Adsorption of Copper (II) Ions from Aqueous Solution by Calcium Carbonate," International Journal of Engineering Research and Technology, vol. 4, no. 12, pp. 413–418, 2015.
[9] Y. D. Ngapa dan J. Gago, "Optimizing of Competitive Adsorption Methylene Blue and Methyl Orange using Natural Zeolite from Ende-Flores," JKPK (Jurnal Kimia dan Pendidikan Kimia), vol. 6, no. 1, pp. 39–48, 2021.
[10] S. Wahyuni, R. Selvina, R. Fauziyah, H. T. Prakoso, P. Priyono, dan S. Siswanto, "Optimasi Suhu dan Waktu Deasetilasi Kitin Berbasis Selongsong Maggot (Hermetia ilucens) Menjadi Kitosan," Jurnal Ilmu Pertanian Indonesia, vol. 25, no. 3, pp. 373–381, 2020. doi: 10.18343/jipi.25.3.373.
[11] A. Waśko, A. Bulak, A. Polak-Berecka, M. Nowakowicz-Dębek, dan A. Bieganowski, "The first report of the physicochemical structure of chitin isolated from Hermetia illucens," International Journal of Biological Macromolecules, vol. 92, pp. 316–320, 2016. doi: 10.1016/j.ijbiomac.2016.07.040.
[12] S. Siddiqui, M. R. Khan, dan M. A. Khan, "The potential of chitin and chitosan from dead black soldier fly (BSF) (Hermetia illucens) for biodegradable packaging material–A critical review," Process Safety and Environmental Protection, vol. 175, pp. 1–13, 2024. doi: 10.1016/j.psep.2024.01.012.
[13] Y. Lin, Y. Zhang, dan X. Chen, "Sustainable extraction of chitin from spent pupal shell of black soldier fly," Processes, vol. 9, no. 6, p. 976, 2021. doi: 10.3390/pr9060976.
[14] T. Hahn, J. Roth, R. Reichl, dan J. Müller, "Purification of chitin from pupal exuviae of the black soldier fly," Waste and Biomass Valorization, pp. 1–16, 2022. doi: 10.1007/s12649-022-01678-0.
[15] N. Abidin, M. A. Kamarudin, dan M. A. Zainol, "The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources," International Journal of Molecular Sciences, vol. 21, no. 3, p. 1–25, 2020. doi: 10.3390/ijms21031020.
[16] R. Forutan, M. R. Khosravi, dan M. R. Khosravi, "The first report of the physicochemical structure of chitin isolated from Hermetia illucens," International Journal of Biological Macromolecules, vol. 92, pp. 316–320, 2016. doi: 10.1016/j.ijbiomac.2016.07.040.
[17] N. Jaafarzadeh, M. A. Rahmanian, dan M. R. Khosravi, "Adsorption of Zn(II) from aqueous solution by using chitin extracted from shrimp shells," Jentashapir Journal of Health Research, vol. 5, no. 11, pp. 131–139, 2013.
[18] Z. Zango, M. R. Khosravi, dan M. R. Khosravi, "A systematic review on applications of biochar and activated carbon derived from biomass as adsorbents for sustainable remediation of antibiotics from pharmaceutical wastewater," Journal of Water Process Engineering, vol. 67, p. 106186, 2024. doi: 10.1016/j.jwpe.2024.106186.
[19] A. Labidi, M. R. Khosravi, dan M. R. Khosravi, "Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies," Journal of the Taiwan Institute of Chemical Engineers, vol. 6, no. 1, pp. 140–148, 2016.
[20] P. Souza, M. R. Khosravi, dan M. R. Khosravi, "Removal of bromophenol blue anionic dye from water using a modified exuviae of Hermetia illucens larvae as biosorbent," Environmental Monitoring and Assessment, vol. 192, no. 3, 2020.
[21] I. Mandasari dan A. Purnomo, “Penurunan Ion Besi (Fe) dan Mangan (Mn) dalam Air dengan Serbuk Gergaji Kayu Kamper,” Jurnal Teknik ITS, vol. 5, no. 1, pp. 1–6, 2016. [22] N. L. Bih et al., “Adsorption of phenol and methylene blue contaminants onto high-performance catalytic activated carbon from biomass residues,” Heliyon, vol. 11, no. 1, p. e41150, 2025. doi: 10.1016/j.heliyon.2024.e41150
[23] T. M. Thanh et al., “Synthesis of iron doped zeolite imidazolate framework‐8 and its remazol deep black RGB dye adsorption ability,” Journal of Chemistry, vol. 2017, Article ID 5045973, 2017. doi: 10.1155/2017/5045973
[24] A. Aichour dan H. Zaghouane-Boudiaf, “Highly brilliant green removal from wastewater by mesoporous adsorbents: Kinetics, thermodynamics and equilibrium isotherm studies,” Microchemical Journal, vol. 146, pp. 1255–1262, 2019. doi: 10.1016/j.microc.2019.02.003
[25] M. Mushtaq et al., “Highly brilliant green removal from wastewater by mesoporous adsorbents: Kinetics, thermodynamics and equilibrium isotherm studies,” Microchemical Journal, vol. 146, pp. 1255–1262, 2019. doi: 10.1016/j.microc.2019.02.003
[26] B. I. Okolo et al., “Adsorption of lead(II) from aqueous solution using Africa elemi seed, mucuna shell and oyster shell as adsorbents and optimization using Box–Behnken design,” Applied Water Science, vol. 10, no. 8, pp. 1–23, 2020. doi: 10.1007/s13201-020-01242-y
[27] K. Phiwdang et al., “Synthesis of CuO nanoparticles by precipitation method using different precursors,” Energy Procedia, vol. 34, pp. 740–745, 2013. doi: 10.1016/j.egypro.2013.06.822
[28] Y. Chuang et al., “Sorption studies of Pb(II) onto montmorillonite clay,” IOP Conference Series: Earth and Environmental Science, vol. 1087, no. 1, p. 012015, 2022. doi: 10.1088/1755-1315/1087/1/012015
[29] Y.-S. Lin et al., “Sustainable extraction of chitin from spent pupal shell of black soldier fly,” Processes, vol. 9, no. 6, p. 976, 2021. doi: 10.3390/pr9060976
[30] M. Zhao et al., “Preparation of honeycomb-structured activated carbon–zeolite composites from modified fly ash and the adsorptive removal of Pb(II),” ACS Omega, vol. 7, no. 1, pp. 9684–9689, 2022. doi: 10.1021/acsomega.1c07000
[31] S. Kim, J. Lee, dan H. Kim, "Identification of pH-dependent removal mechanisms of lead and arsenic by basic oxygen furnace slag: Relative contribution of precipitation and adsorption," Journal of Cleaner Production, vol. 279, p. 123451, 2021. doi: 10.1016/j.jclepro.2020.123451
[32] N. Zainudin, R. I. M. Rashid, A. R. Ishak, dan A. Ali, "Removal of Lead (Pb) From Aqueous Solutions Using Exoskeleton of Black Soldier Fly (BSF)," Environment-Behaviour Proceedings Journal, vol. 8, no. 25, pp. 175–184, 2023. doi: 10.21834/ebpj.v8i25.4864
[33] X. Li, L. Xu, J. Gao, M. Yan, H. Bi, dan Q. Wang, "Surface modification of chitin nanofibers with dopamine as efficient nanosorbents for enhanced removal of dye pollution and metal ions," International Journal of Biological Macromolecules, vol. 253, p. 127113, 2023. doi: 10.1016/j.ijbiomac.2023.127113
[34] A. Basem, M. A. El-Sayed, dan H. A. El-Sayed, "Adsorption of heavy metals from wastewater by chitosan: A review," Results in Engineering, vol. 19, p. 102404, 2024. doi: 10.1016/j.rineng.2023.102404
[35] D. Nasution, R. N. Lubis, dan R. S. Lubis, "Determination of Maximum Adsorption Capacity of Chitosan and Carboxymethyl Chitosan on the Absorption of Metal Ions Cr (VI) Based on the Langmuir Equation," Journal of Chemical Natural Resources, vol. 6, no. 1, pp. 30–38, 2024.
[36] K. L. Tan dan B. H. Hameed, "Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions," Journal of the Taiwan Institute of Chemical Engineers, vol. 74, pp. 25–48, 2017. doi: 10.1016/j.jtice.2017.01.024
[37] D. Chicco, M. J. Warrens, dan G. Jurman, "The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation," PeerJ Computer Science, vol. 7, p. e623, 2021. doi: 10.7717/peerj-cs.623
[38] A. M. Alwan Al Mashhadani, T. A. Himdan, A. S. Hamadi Al Dulaimi, dan Y. I. M. AbuZaid, "Adsorptive removal of some carbonyl containing compounds from aqueous solutions using Iraqi porcelanite rocks: a kinetic-model study," Caspian Journal of Environmental Sciences, vol. 20, no. 1, pp. 117–129, 2022. doi: 10.22124/cjes.2022.5406
[39] P. Rahmania dan A. Bagastyo, "Removal of napthol dye from batik wastewater using chitosan from black soldier fly pupal exuviae," Analit: Analytical and Environmental Chemistry, vol. 9, no. 1, pp. 151–163, 2024. T
[40] Y. Chuang, J. Chen, J. Lu, L. Su, S. Y. Jiang, Y. Zhao, C. H. Lee, Z. Wu, dan H. D. Ruan, "Sorption studies of Pb(II) onto montmorillonite clay," IOP Conference Series: Earth and Environmental Science, vol. 1087, no. 1, p. 012007, 2022. doi: 10.1088/1755-1315/1087/1/012007
[41] J. Algethami, M. A. Alharthi, M. A. Alshahrani, dan M. A. Alharthi, "Chitin extraction from crab shells and synthesis of chitin@metakaolin composite for efficient amputation of Cr(VI) ions," Environmental Research, vol. 252, p. 119065, 2024. doi: 10.1016/j.envres.2021.119065
[42] M. Abomosallam, M. A. Alharthi, M. A. Alshahrani, dan M. A. Alharthi, "Adsorption kinetics and thermodynamics of toxic metal ions onto chitosan nanoparticles extracted from shrimp shells," Nanotechnology for Environmental Engineering, vol. 7, pp. 1–13, 2022. doi: 10.1007/s41204-022-00145-2
[43] M. Makhlouf, M. A. Alharthi, M. A. Alshahrani, dan M. A. Alharthi, "Eco-friendly synthesis of biosorbent based on chitosan-activated carbon/zinc oxide nanoparticle beads for efficiency reduction of cadmium ions in wastewater," Biomass Conversion and Biorefinery, pp. 1–16, 2024. doi: 10.1007/s13399-024-04567-2
[44] A. A. Alwan Al Mashhadani, T. A. Himdan, A. S. Hamadi Al Dulaimi, dan Y. I. M. AbuZaid, "Adsorptive removal of some carbonyl containing compounds from aqueous solutions using Iraqi porcelanite rocks: a kinetic-model study," Caspian Journal of Environmental Sciences, vol. 20, no. 1, pp. 117–129, 2022. doi: 10.22124/cjes.2022.5406
[45] A. Tomczyk, M. S. Sokołowska, dan M. Boguta, "Biomass type effect on biochar surface characteristic and adsorption capacity relative to silver and copper," Fuel, vol. 278, p. 118183, 2020. doi: 10.1016/j.fuel.2020.118183
Refbacks
- There are currently no refbacks.








